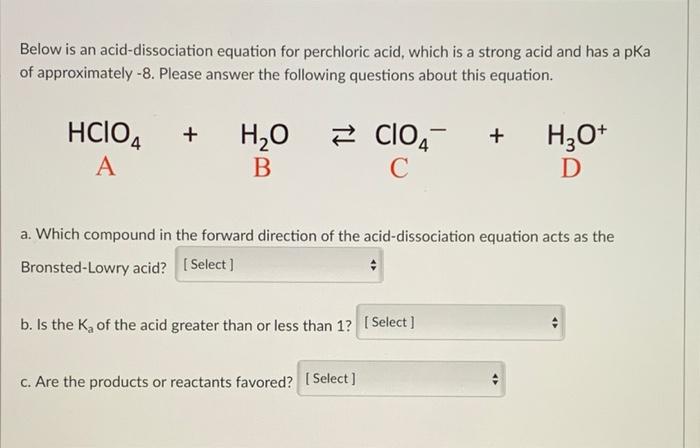
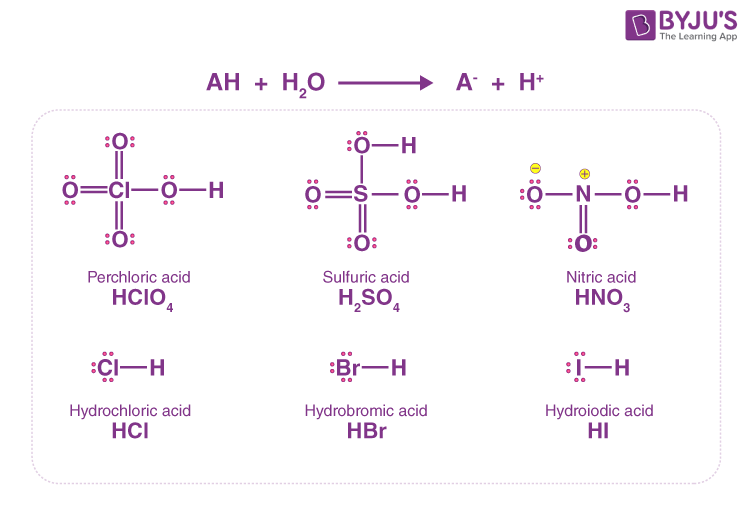
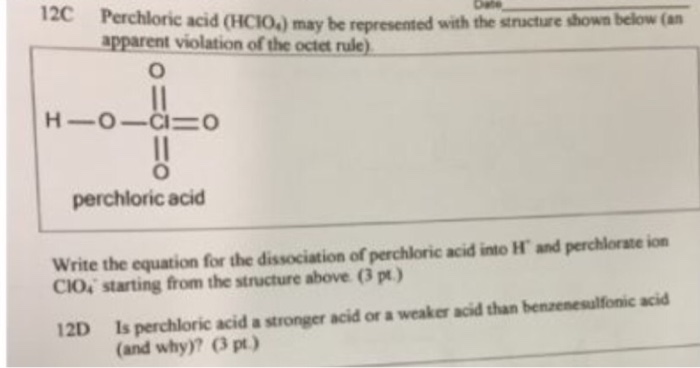
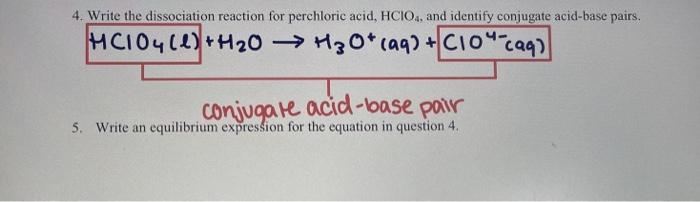
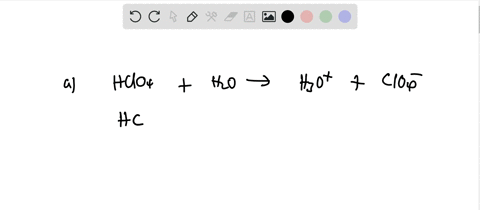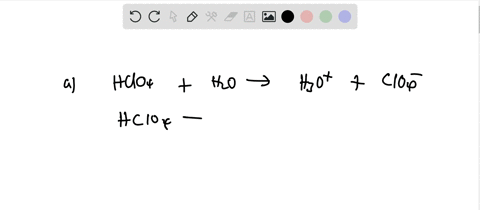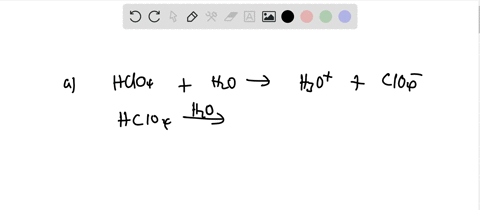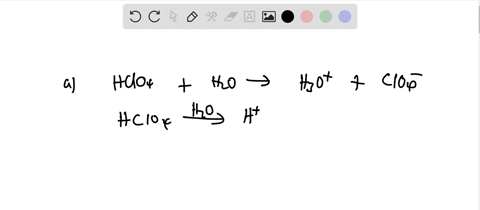Ionization of Acid And Bases - Arrhenius concept of Acid And Base Ionisation, Explanation, Determination ionisation constant of Acid base, Examples And FAQS

Dissociation Constants of Perchloric and Sulfuric Acids in Aqueous Solution | The Journal of Physical Chemistry B

Strong Acids Ions are present in an aqueous solution of an acid, because these ions result from the dissociation of the acid. An acid that dissociates. - ppt download

Difference Between Perchloric Acid and Hydrochloric Acid | Compare the Difference Between Similar Terms

Dissociation of perchloric acid measured by Raman and n.m.r. spectrometry - Chemical Communications (London) (RSC Publishing)

Representative in vitro 31 P-NMR spectra (perchloric acid extracts,... | Download Scientific Diagram

Electrochemical formation and reactivity of a manganese peroxo complex: acid driven H 2 O 2 generation vs. O–O bond cleavage - Chemical Science (RSC Publishing) DOI:10.1039/C3SC53469C