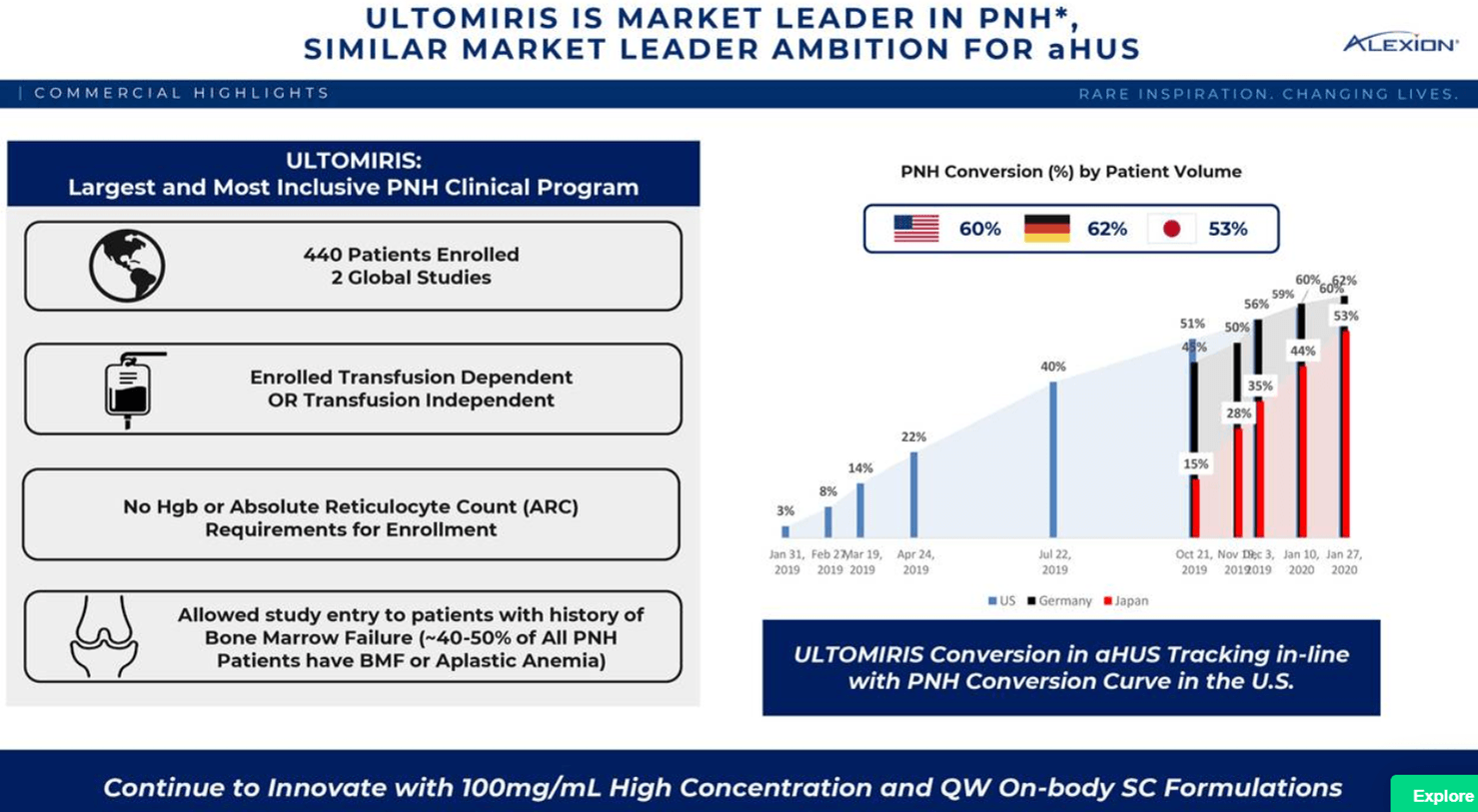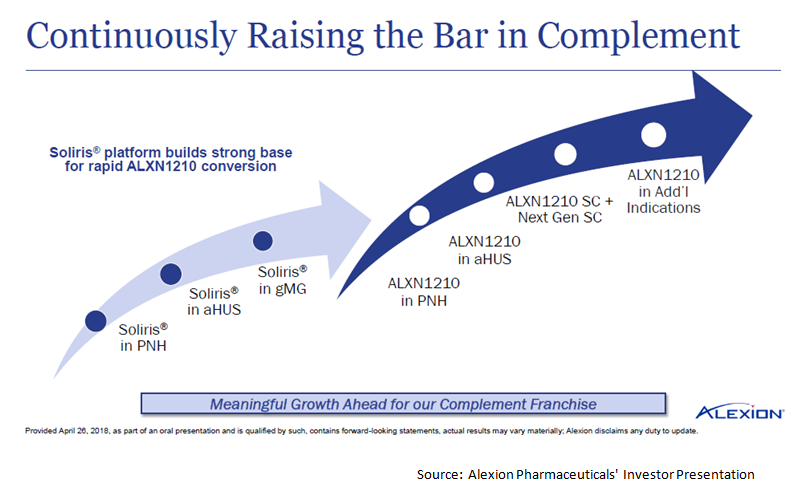
Alexion Pharmaceuticals' Diversification Strategy Makes It A Solid Buy For 2018 (NASDAQ:ALXN) | Seeking Alpha

Alexion Receives FDA Approval for New Advanced Formulation of ULTOMIRIS® (ravulizumab-cwvz) with Significantly Reduced Infusion Time | Business Wire
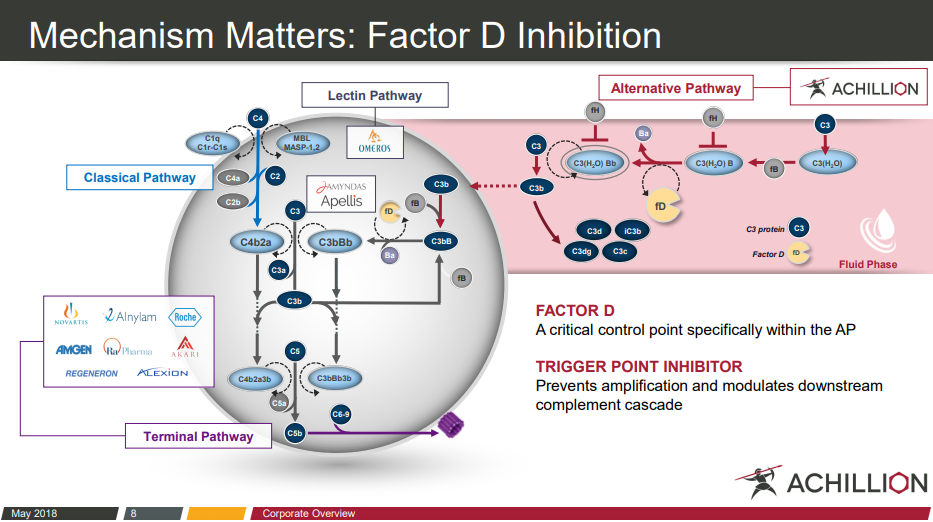
Alexion Pharmaceuticals: Clinical Progress, Acquisitions And The Competition (Part 2) (NASDAQ:ALXN) | Seeking Alpha
Alexion Announces FDA Approval of ULTOMIRIS® (ravulizumab-cwvz) for Children and Adolescents with Paroxysmal Nocturnal Hemoglobinuria (PNH) | Alexion Pharmaceuticals, Inc.

Alexion's 'Meh' 2017 Guide Soothes Worries Of Decline, But PNH Drug Key | Stock News & Stock Market Analysis - IBD